Nanotechnology and Carbon
“There’s Plenty of Room at the Bottom” was a lecture given by Richard Feynman a physics Professor at Caltech in 1959. He described a future where we would be able to control individual atoms. By doing so, we would be able to create new materials, discover new medicines, and in fact, create a brighter, stronger and robust future for mankind. People look back at this lecture as one of the first descriptions of what we know today as nanotechnology.
What is a nanometer?
One nanometer (nm) is 1 billionth of a meter, or 0.000000001 meters. To put that into perspective, you would have to slice a human hair 50,000 times to reach 1 nanometer. Another way to see this is to compare the size of a marble to the earth. If 1 nm were the diameter of a marble, then 1 meter would be the diameter of the earth.
Over the years we have learned that materials behave differently at this scale; for example, metals will melt at much lower temperatures given that their surface area is greatly enhanced. Today we see this ability being applied in 3D printers. Carbon, in its nanotube form, is 100 times stronger than steel and the basis of very high strength composite materials.
How is carbon related to nanotechnology?
Carbon is a common element. Its forms include diamond, graphite, coal, activated carbon, and carbon black. Millions of tons of these materials are used every year in applications that include abrasives, tires and water treatment, among others.
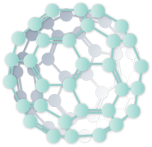
A fullerene is a highly unique form of carbon, a molecule at the nm scale. Fullerenes were first “discovered” in 1985 by Richard Smalley and colleagues at Rice University, although as a molecular material the credit would go to E.G. Osawa, a Japanese chemist, who first proposed its existence a decade earlier. Given its resemblance to the geodesic dome created by Buckminster Fuller, it became known as the Buckminster Fullerene, or Fullerene. This commonly known Fullerene has 60 carbon atoms (“C60”); later discoveries led to fullerene structures with 70 carbon atoms and others. The diameter of a C60 fullerene is 0.7 nanometers. Its properties range from:
- Superconductor to semi-conductor
- Radical scavenger
- Extreme durability
- Easily modified to tailor its properties
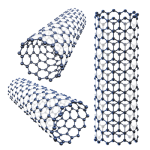
Single Walled Carbon Nanotubes (SWCNT), another new form of carbon, were discovered in 1993. The discovery was published in the journal Nature by two groups working independently (Ijima and Bethune). A SWCNT ranges in diameter from 0.9 nanometers to 1.5 nanometers, with typical lengths ranging from 500 to over 2,000 nanometers. It can be
- A semi-conductor or metal
- Stronger than steel, yet lighter than aluminum
- Most efficient conductor of heat
- Easily modified to tailor properties as an “ink”
Why does it matter?
It is perhaps surprising to recognize that carbon provides a foundation for addressing some of the most serious challenges facing our world:
- Demands for alternatives to fossil fuels (energy security, global warming)
- Drive for efficiency (extend end of life, limit use of exotic materials, energy efficiency)
- Access to clean water and air (groundwater, desalination)
- Needs of an aging population (health care and advanced therapeutics)
For example, lithium ion batteries used in electric vehicles won’t work without carbon, modern-day water filtration systems need carbon to capture impurities, materials processing depends on carbon for lubrication, and synthetic diamonds used to protect materials in erosive industrial environments are made from carbon.
The new class of carbon materials and chemicals that Nano-C manufactures a will have a greater and more profound effect than existing forms of carbon in shaping solutions to the four challenges listed above. Nano-C’s innovations are derived from its competency in the chemistry of these new forms of carbon, enabling its customers to:
- Shrink the size of next generation semiconductors
- Make flash memory more energy efficient
- Provide heavy-metal free electrodes for displays
- Create low-cost organic electronic and medical devices
- Develop low-cost solar cells
- Develop lighter weight/lower cost fuel cells for transportation
- Make batteries that can be printed
- Create water filters that won’t foul
- Capture free radicals before they can damage cells
- Create X-ray sensors that limit radiation exposure
These highly advanced materials are at the foundation of many more game-changing applications. Indeed, the opportunity in nano-structured carbon is anything but nano.